Negative contrast electron microscopy of purified virus and thin section electron microscopy of the brain of an infected hamster and the salivary gland of an infected fox. |
| | |
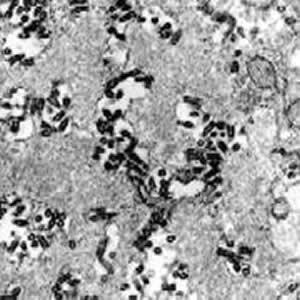 | Rabies virus infection, hamster brain. This micrograph covers just part of the cytoplasm of an infected neuron. Two hallmarks of rabies virus infection are seen — there is minimal damage seen to the structure of infected neurons even though the extent of the infection is dramatic, and large numbers of bullet-shaped virions accumulate as a result of budding upon the endoplasmic reticulum membranes of these cells. Magnification approximately x25,000.
Micrograph from F. A. Murphy, University of Texas Medical Branch, Galveston, Texas. Download TIF |
| | |
 | Rabies virus infection, hamster brain. This is the same image as above, but colorized. This micrograph covers just part of the cytoplasm of an infected neuron. Two hallmarks of rabies virus infection are seen — there is minimal damage seen to the structure of infected neurons even though the extent of the infection is dramatic, and large numbers of bullet-shaped virions accumulate as a result of budding upon the endoplasmic reticulum membranes of these cells. Magnification approximately x25,000.
Micrograph from F. A. Murphy, University of Texas Medical Branch, Galveston, Texas. Download TIF |
| | |
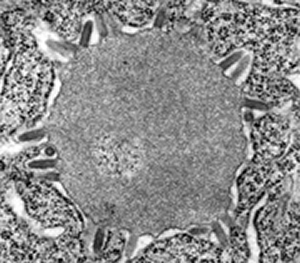 | Rabies virus infection, hamster brain, showing an early stage in the formation of a Negri body, as the massed excess viral nucleocapsid material condenses into the dense form that when larger may be seen at the light miroscopic level of magnification. Here, some virions are seen budding from intracytoplasmic membranes surrounding the Negri body. Magnification approximately x30,000. Micrograph from F. A. Murphy, University of Texas Medical Branch, Galveston, Texas. Download TIF |
| | |
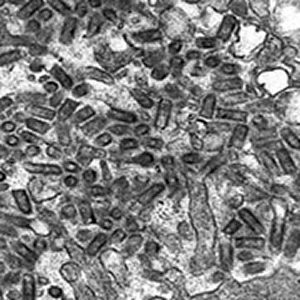 | Rabies virus in the salivary gland of a rabid fox. In this image there are salivary gland cells (cells that make mucous saliva) on both edges. The salivary space in the center, which leads downstream to the salivary duct, is filled with bullet-shaped virions. The virions are sectioned in various planes so they do not all look like bullets. Magnification: approximately x40,000.
Micrograph from F. A. Murphy, University of Texas Medical Branch, Galveston, Texas. Download TIF |
| | |
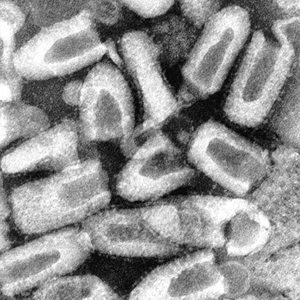 | Rabies virus, purified from an infected cell culture. Negatively stained virions: note their characteristic "bullet shape." Magnification approximately x70,000.
Micrograph from F. A. Murphy, University of Texas Medical Branch, Galveston, Texas. Download TIF |
| | |
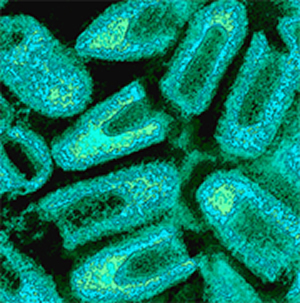 | Rabies virus. The same image as above, but colorized. Virus purified from an infected cell culture. Negatively stained virions: note their characteristic "bullet shape." Magnification approximately x70,000.
Micrograph from F. A. Murphy, University of Texas Medical Branch, Galveston, Texas. Download TIF |
| | |
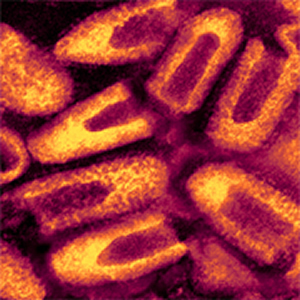 | Rabies virus. The same image as above, but colorized. Virus purified from an infected cell culture. Negatively stained virions: note their characteristic "bullet shape." Magnification approximately x70,000.
Micrograph from F. A. Murphy, University of Texas Medical Branch, Galveston, Texas. Download TIF |
| | |
 | Rabies virus. Diagnostic immunofluorescence, fox brain, 1965. The direct immunofluorescence method was first applied to rabies diagnosis by Robert Goldwasser and Robert Kissling at CDC in 1958 (Goldwasser RA and Kissling RE. Fluorescent antibody staining of street and fixed rabies virus antigens. Proc Soc Exp Biol Med 98:219-223, 1958). I was in the U.S. Army Veterinary Corps at the Fourth U.S. Army Medical Laboratory, Fort Sam Houston, Texas, in 1959, which was the first laboratory to employ the new method in the field -- there was lots of rabies in the area then and lots of diagnostics to do. The method and reagents were just as good from the beginning as they are now: fluorescent microscopes are better and monoclonal antibody-based fluorescent conjugates are often used, but the sensitivity and specificity and practicality of the method have not changed. The image here is of a touch impression from the brainstem of a rabid fox, stained with goat anti-rabies hyperimmune globulin, conjugated with fluorescein-isothiocyanate, and examined under deep ultraviolet light with filters allowing only the wavelength of the specific fluorescence to be seen (along with enough background to see the specimen -- here brown, but different in different setups). Magnification approximately x250.
Micrograph from F. A. Murphy, University of Texas Medical Branch, Galveston, Texas. Download TIF |