The Menon Lab Perinatal Research Lab
Senescence of feto-maternal interface tissues
STUDY TOPICS
- Telomere dependent physiologic and pathologic aging of fetal membranes.
- Fetal membrane senescence as a mechanistic contributor of term and preterm births.
- Oxidative stress-induced p38 mitogen-activated kinase activation (MAPK) as a mechanistic modulator of fetal membrane senescence.
- Oxidative stress and oxidative stress-induced damage products as activators of p38MAPK signaling in term and preterm birth.
- Pathways p38MAPK activation in human fetal membrane cells.
Senescence and senescence-associated secretory phenotype (SASP) in preterm and term birth. - Molecular differences in senescence activation by infectious and oxidative stress signals in feto-maternal interface cells.
- Mechanistic differences in the activation of infectious and sterile inflammation in fetal membranes.
- Understanding the type of cell deaths in the fetal membrane and placental cells in response to pregnancy-associated risk factors.
Using in vitro models of tissue explants (developed by Dr. Menon), primary cell cultures, and animal models the lab studies fetal contributions to initiation of parturition as well as various pregnancy-associated complications; specifically spontaneous preterm birth and preterm premature rupture of the fetal membranes.
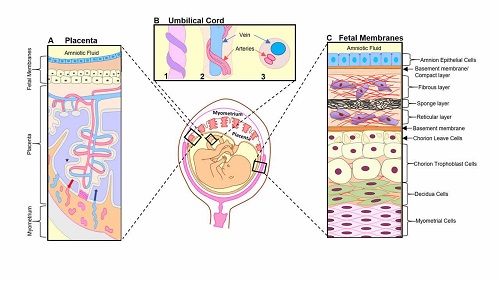
Senescence in human and mouse fetal membrane tissues are telomere dependent and p38 mitogen activated kinase (MAPK) mediated process.
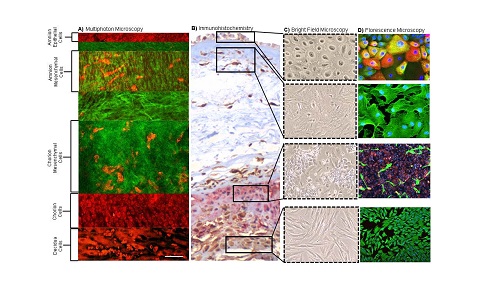
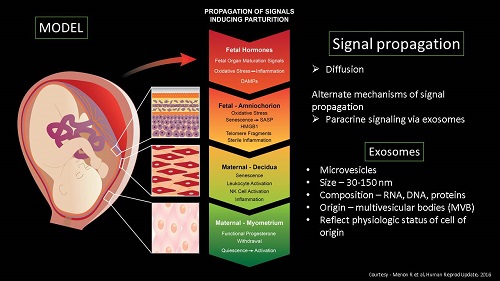
Senescent cells release senescence-associated secretory phenotype (SASP) and damage associated molecular pattern (DAMP) molecules via extracellular vesicles (exosomes) to communicate with maternal uterine tissues. This 'sterile inflammatory' communication results in increased inflammatory load leading to labor and delivery.
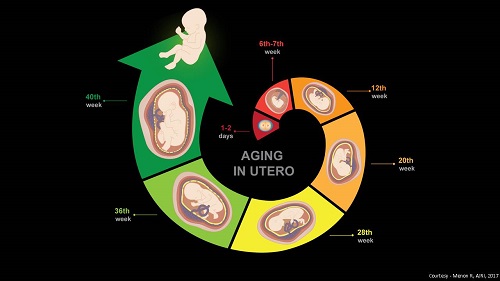
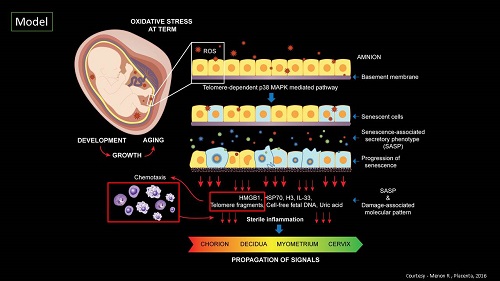
The Menon lab hypothesizes that premature activation of senescence cascade in response to adverse pregnancy conditions (e.g. infection, environmental pollutants, various stress factors, behavioral or nutritional issues, mechanical trauma) produces premature fetal membrane aging and a dysfunctional status for membranes. A factor that can contribute to preterm birth and preterm premature rupture of the membranes. The lab is also interested in designing approaches to reduce preterm birth risk by regulating p38MAPK activities in fetal membrane cells by slowing down senescence.
 Exosomes
– A novel therapeutic approach to treat preterm birth by inhibiting the NF-κB pathway project
Exosomes
– A novel therapeutic approach to treat preterm birth by inhibiting the NF-κB pathway project
Leader: Ananth Kumar Kammala and Enktuya Radnaa, PhD
A major contributor to preterm birth (PTB) is infection and the subsequent host inflammatory response. Our lab has shown that fetal innate immune cells play an important role in the response to infection, specifically with respect to histologic chorioamnionitis
(infiltration of
 neutrophil
to the fetal membranes), which is a fetal, not maternal, neutrophil response. Therapeutics is currently being developed to minimize the inflammatory responses mediated by the pro-inflammatory transcription factor NF-κB. However, despite promising
in situ and in vitro results, very few have made it to clinical trials, and none are in-use clinically due to their inability to cross the placenta or toxicity to fetal growth. Extracellular vesicles (EV) have emerged as potential drug delivery vehicles
since they are inert, non-immunogenic, and natural cell-derived nanoparticles that we and others have shown can cross the placenta barrier. Therefore, in collaboration with ILIAS Biologics in Daejeon, South Korea, we have engineered EVs to contain
an inhibitor to the NF-κB pathway called Super Repressor IκBα (SR, Figure 1). SR is a mutant form of IκBα that can inhibit NF-κB function. Preliminary studies in our lab indicate SR can delay infection-induced
PTB so we are now performing preclinical studies to determine efficacy, dosing, kinetics, and biodistribution.
neutrophil
to the fetal membranes), which is a fetal, not maternal, neutrophil response. Therapeutics is currently being developed to minimize the inflammatory responses mediated by the pro-inflammatory transcription factor NF-κB. However, despite promising
in situ and in vitro results, very few have made it to clinical trials, and none are in-use clinically due to their inability to cross the placenta or toxicity to fetal growth. Extracellular vesicles (EV) have emerged as potential drug delivery vehicles
since they are inert, non-immunogenic, and natural cell-derived nanoparticles that we and others have shown can cross the placenta barrier. Therefore, in collaboration with ILIAS Biologics in Daejeon, South Korea, we have engineered EVs to contain
an inhibitor to the NF-κB pathway called Super Repressor IκBα (SR, Figure 1). SR is a mutant form of IκBα that can inhibit NF-κB function. Preliminary studies in our lab indicate SR can delay infection-induced
PTB so we are now performing preclinical studies to determine efficacy, dosing, kinetics, and biodistribution.
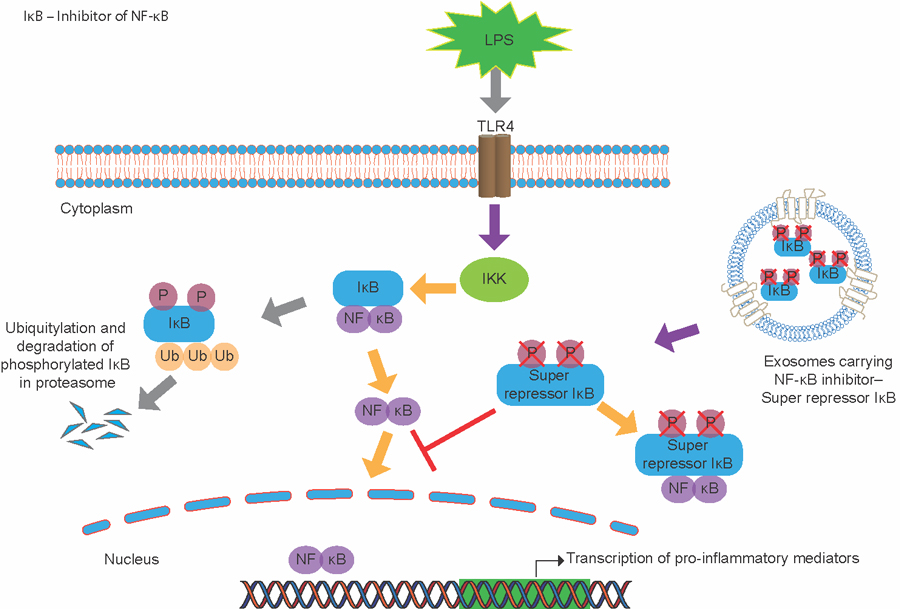
Figure 1: Schematic of NF-κB activation and proposed mechanism of super repressor (SR) in cells.
In the cell, NF-κB is sequestered to the cytoplasm by its biological inhibitor IκB. In the presence of a pro-inflammatory stimulus, IκB kinase is activated and phosphorylates IκB.
This leads to its polyubiquitination and degradation and allows NF-κB to translocate to the nucleus to initiate the transcription of pro-inflammatory mediators. SR is a mutant form of IκB that cannot be phosphorylated by IκB
kinases. By packaging SR into exosomes, we can effectively deliver SR to cells and tissues and suppress the translocation of the NF-κB complex to the nucleus, even in the presence of a pro-inflammatory stimulus, such as lipopolysaccharide
(LPS).
 Engineering
HMGB1 in exosomes to determine feto-maternal signaling Project leader: Enktuya Radnaa, PhD
Engineering
HMGB1 in exosomes to determine feto-maternal signaling Project leader: Enktuya Radnaa, PhD
High mobility group box (HMGB)1, one of the damage-associated molecular pattern (DAMPs) markers, amplifies immune responses within the gestational tissues and plays an important role in spontaneous preterm birth. However, the molecular mechanism of inflammation
induced by HMGB1 is not clear. In our lab, we found that HMGB1 is packaged inside the exosomes released by senescent fetal amnion membrane cells of the placenta. Our current hypothesis is that the exosomes containing HMGB1 enhance the inflammatory
load at local (fetal membrane), as well as distant (maternal) uterine tissues. Moreover, we are testing the trafficking of HMGB1 containing exosomes between cellular layers of the fetal membrane and its ability to cause inflammation. We are currently
engineering primary amnion epithelial cells (AECs)-derived exosomes to contain HMGB1 to test its function on maternal uterine cells. We are using an Organ-On-Chip (OOC) system to recreate the fetal membrane in vitro. Our preliminary data show that
rhHMGB1-induced inflammatory signal propagated through the fetal membrane cells to maternal cells in the OOC devices.
Microvesicles from fetal membrane cells – characterization and function Project leader:
Extracellular vesicles (EVs) have been identified for their key role in intercellular communication and propagation of signals during normal and pathological conditions. One of the major mechanisms of feto-maternal cross-talk during pregnancy and parturition is through extracellular vesicles (EVs) such as exosomes (EXs, 30-150 nm) and microvesicles (MVs, 160- 1000nm). Thus, insights into fetal membrane-derived EVs open a novel approach in understanding some of the major pregnancy-related complications such as preterm birth. In this context, we have been working on studying microvesicles released from the fetal membrane at term. MVs are large heterogeneous group of extracellular vesicles found in various biological fluids. They are released from the surface of the cell membrane by outward budding and fission. Thus, their composition and function depend primarily on their origin. Our aim is to fully characterize isolated MVs from cell culture through identification of their protein cargo and specific biomarkers, morphology, functional role in feto-maternal communication, and potential pro-inflammatory effect on maternal cells such as myometrial, decidual, and cervical cells.
Currently developed OOCs mimicking components of the fetal membranes and feto-maternal interfaces Project Leader:
(PDF of paper available here).
The Menon lab is also investigating how these
signals are regulated during normal pregnancy and how they contribute to fetal
membrane homeostasis. The Menon lab has recently discovered 'microfractures' in
the fetal membranes (please see video), a structural feature associated with
fetal membrane remodeling. Microfractures are sites of tissue remodeling sites
where amnion epithelial cells undergo epithelial mesenchymal transitions (EMT).
The Menon lab is investigating mechanisms of EMT and recycling capabilities
(MET) of amniochorion cells (Figure 1; PDF of paper available). Using an
Organ-On-Chip (OOC) approach (Figure 2), The Menon lab is studying the
microenvironments associated with fetal membrane remodeling (Figure 2 A-C; Richardson
et al. FASEB, 2019) and its dysfunction in response to various pregnancy
associated risk factors (Figure 2 D-F; Richardson et al. Reproductive
Sciences, 2019).

Using an Organ-On-Chip (OOC) approach (Figure 2), The Menon lab is studying the microenvironments associated with fetal membrane remodeling (Figure 2 A-C; Richardson et al. FASEB, 2019) and its dysfunction in response to various pregnancy-associated risk factors (Figure 2 D-F; Richardson et al. Reproductive Sciences, 2019).”
Schematic of amnion membrane maintenance and disruption due to cellular transitions Project Leader:

Detection of phosphatidyl serine in the membrane of microvesicles by staining with Annexin-V-FITC (left). Transmission Electron Microscopy (TEM) showing the morphology and size of microvesicles isolated from amnion epithelial cells (right).

Role of exosomes in Ureaplasma spp. transmission and ascending intra-amniotic infection Project leader: Ourlad Alzeus Tantengco, MD
Ureaplasma spp. infection is associated with preterm labor. However, the exact mechanisms how Ureaplasma spp. infect cells in the female genital tract, establish ascending infection and colonize the amniotic cavity are yet to be fully elucidated.
 I hypothesize that exosomes from cervical epithelial cells infected with Ureaplasma spp. serve as a carrier for the transmission and establishment of ascending infection by Ureaplasma spp. in fetal membranes and placenta. Using the traditional cell culture, organ-on-chip, and tissue explants, I will investigate if these extracellular vesicles can cause epithelial-mesenchymal transition, inflammatory response, and cell death in the cervix and the fetal membranes. These mechanisms are involved in premature cervical remodeling and fetal membrane weakening which are among the major mechanisms leading to preterm labor. Aside from these, I am also studying the role of steroid hormones, progesterone, and β-estradiol, on epithelial-mesenchymal transition in cervical epithelial and stromal cells.
I hypothesize that exosomes from cervical epithelial cells infected with Ureaplasma spp. serve as a carrier for the transmission and establishment of ascending infection by Ureaplasma spp. in fetal membranes and placenta. Using the traditional cell culture, organ-on-chip, and tissue explants, I will investigate if these extracellular vesicles can cause epithelial-mesenchymal transition, inflammatory response, and cell death in the cervix and the fetal membranes. These mechanisms are involved in premature cervical remodeling and fetal membrane weakening which are among the major mechanisms leading to preterm labor. Aside from these, I am also studying the role of steroid hormones, progesterone, and β-estradiol, on epithelial-mesenchymal transition in cervical epithelial and stromal cells.
Specific interests:
- Senescence of fetal membranes and regulation of senescence to reduce preterm birth.
- Feto-maternal communications via exosomes.
- Feto-maternal interface microenvironment regulation by exosomes and their functional contributions in pregnancy and parturition.
- Microarchitecture of human fetal membranes, its function during pregnancy and mechanisms of membrane remodeling.
- Redefining the role of infectious agents and inflammatory
mediators induced by infectious agents in fetal membranes during preterm
birth.
- Contributions of environmental pollutants in preterm birth.
- Image and exosome based biomarkers of preterm birth and preterm premature rupture of the membranes.