Cyanosis
L. Yun, S. Munir, M.E. Gomez, MD A. Aly, MD, PhD
Definition
Cyanosis is a blue discoloration of the skin and/or mucous membranes. It is due to the presence of greater than 3 g/dL of reduced or deoxygenated Hb (Hb) in the blood. It is important to note that cyanosis is dependent on the absolute concentration of reduced Hb. Cyanosis can be clinically appreciated when the O2 saturation is < 85%.
Effect of Hb level on Cyanosis
At a normal Hb level of 15 g/dL, the presence of 3 g/dL of reduced Hb results in 20% desaturation. Therefore, cyanosis is visible when O2 saturation is approximately ~80%. The lower the Hb level, the lower the O2 saturation needed before cyanosis can be appreciated.
- If the patient has Hb level 21 g/dL, the presence of 3 g/dL of reduced Hb leads to about 15% desaturation (3g/dL per 21 g/dL = 15%). Cyanosis is thus visible when O2 saturation is reduced to 85%.
- Conversely, at a Hb level of 10 g/dL such as in anemia, the presence of 3 g/dL of reduced Hb results in about 30% desaturation (3g/dL per 10 g/dL = 30%) and cyanosis does not appear until O2 saturation is ~70% . By the time cyanosis is noted in a severely anemic patient, the concentration of Hb is markedly reduced and the patient is usually very sick.
Effect of Fetal Hb on Cyanosis
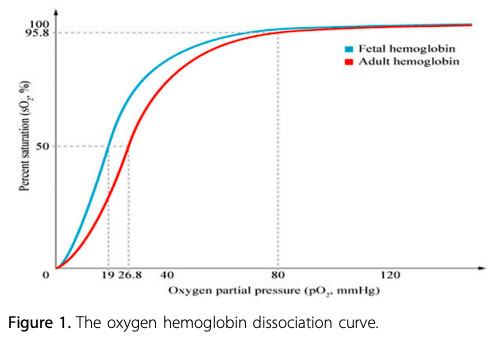
Fetal Hb (HbF) is the main oxygen carrier protein in the human fetus during the last 7 months of pregnancy and persists during the first 6 months after birth. HbF has a single amino acid substitution (histidine to serine) at the 2,3-bisphosphoglycerate (2,3-BPG) binding site, which results in a higher affinity for oxygen than seen in adult Hb. The partial pressure of oxygen (PaO2) at which HbF is 50% saturated (P50) is lower than in adult Hb.
Infants with a higher proportion of HbF may have a greater reduction in oxygenation before cyanosis is clinically apparent. For example, in infants who have mostly adult Hb, central cyanosis (arterial saturation 75% to 85%) will be observed when the PaO2 falls below 50 mmHg. In contrast, in infants with mostly HbF, central cyanosis may not be observed until the PaO2 drops well below 40 mmHg.
Causes of Neonatal Cyanosis
- Respiratory system disorders are the most common causes of cyanosis in neonates and should be ruled out first
- Airway obstruction - choanal atresia, micrognathia, laryngomalacia
- Pulmonary pathology and V/Q mismatch - pneumonia, pulmonary hypoplasia, persistent pulmonary hypertension (PPHN)
- Central nervous system disorders cause depression of respiratory drive
- Neonatal encephalopathy, seizures, intracranial hemorrhage
- Hematologic disorders
- Methemoglobinemia results from the oxidation of Hb molecules from the normal ferrous to the abnormal ferric state. Patients may appear cyanotic without respiratory distress and have a decreased O2 saturation with normal PaO2.
- Polycythemia may cause peripheral cyanosis due to decreased perfusion secondary to hyperviscosity
- Cardiovascular disorders
- Cyanotic congenital heart disease (CCHD): 6 T's
- d-transposition of the great arteries (d-TGA)
- Tetralogy of Fallot
- Truncus Arteriosus
- Total Anomalous Pulmonary Venous Connection
- Tricuspid Atresia
- (The) Hypoplastic Left Heart Syndrome
- Eisenmenger Syndrome: pulmonary hypertension with reversal of a L-to-R shunt
Refer to the section on "Cyanotic Cardiac Lesions" for more detail
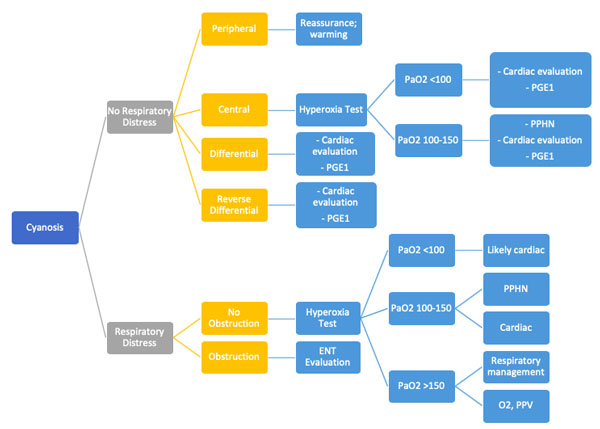
Evaluation of Neonatal Cyanosis
Types of Cyanosis:
- Central Cyanosis
- Seen in the tongue and mucous membranes
- Cause: desaturation of the arterial blood
- Treatment: dependent on the underlying cause
- Peripheral Cyanosis (acrocyanosis, perioral cyanosis)
- Seen in the tips of fingers, toes and around the lips
- Cause: peripheral vasoconstriction (common in patients with Down syndrome due to vasomotor instability); patient will have normal arterial O2 saturation
- Treatment: reassurance, rewarming of extremities
- Differential Cyanosis
- Cyanosis seen only in the lower part of the body
- Causes:
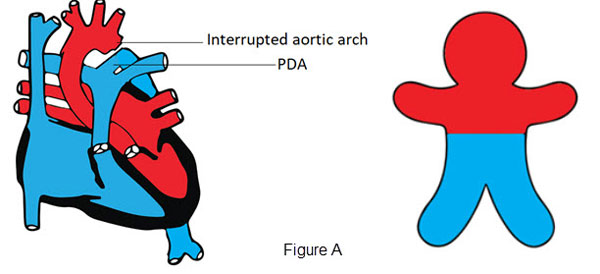
- interrupted aortic arch or critical coarctation of the aorta in the presence of a patent ductus arteriosus (PDA) due to right to left shunt to the lower part of the body (Figure A)
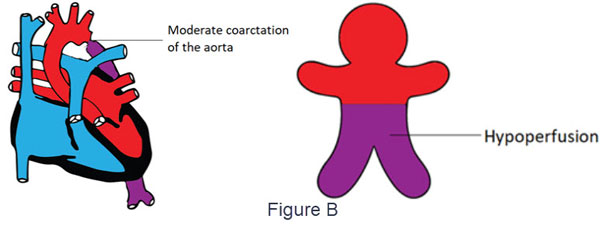
- Moderate coarctation in the absence of PDA may also cause a variable degree of differential cyanosis due to hypoperfusion (Figure B)
- Pulmonary hypertension with a super-systemic pulmonary pressure causing a right to left shunt across a patent ductus arteriosus
- Reverse Differential Cyanosis
- Cyanosis seen only in the upper part of the body
- Causes:
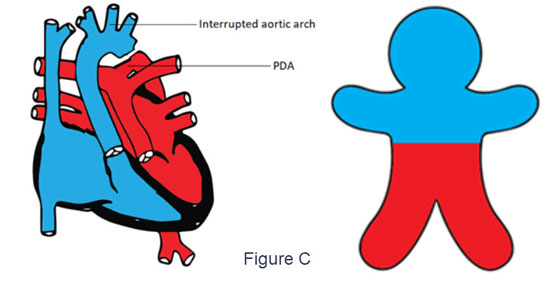
- d-TGA with PDA and severe coarctation of the aorta or interrupted aortic arch (Figure C)
- The deoxygenated blood flows from the right ventricle through the aorta to the upper part of the body cyanosis
- Lower part of the body is perfused with oxygenated blood from the left ventricle, passing through the pulmonary artery and PDA.
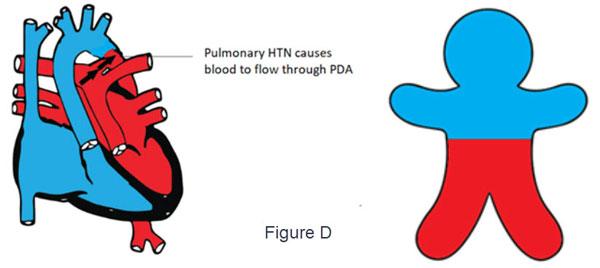
- d-TGA with PDA and suprasystemic pulmonary HTN (Figure D)
- The deoxygenated blood flows from the right ventricle through the aorta to the upper part of the body cyanosis
- Suprasystemic pulmonary pressure shunts oxygenated blood through the PDA to the lower part of the body.
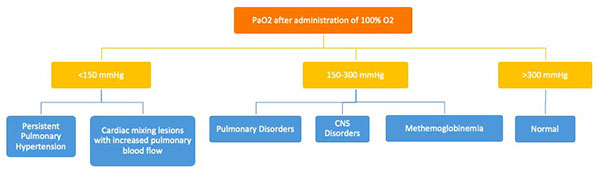
Hyperoxia Test
The hyperoxia test is used to help differentiate between cardiac and noncardiac causes of cyanosis. The PaO2 is measured in the right radial artery (preductal) on room air and after 10 minutes of 100% oxygen supplementation. The hyperoxia test is interpreted as shown in Figure E. Failure to increase O2 saturation and PaO2 raises the suspicion for cyanotic heart disease. In severe forms of lung disease or PPHN, the PaO2 may not increase with the hyperoxia challenge. This test has limited utility in facilities with access to echocardiography but it may guide decision-making for the necessity of prostaglandin infusion.
Role of Critical Congenital Heart Disease (CCHD) Screening in the Cyanotic Neonate:
In CCHD pulse oximetry screening, preductal (right upper extremity) saturations are compared with post-ductal saturations (lower extremity). A failed screening is when:
- Oxygen saturation value is less than 90%
- Oxygen saturation is less than 95% in the right hand and foot on 3 measurements, each separated by an hour
- Difference between pre- and post-ductal saturations is greater than 3% on 3 measurements, each separated by 1 hour
CCHD Screening is effective in early identification in the following 7 primary screening targets:
- Hypoplastic Lleft Hheart Syndrome
- Pulmonary Atresia with an iIntact sSeptum
- Tetralogy of Fallot
- Total aAnomalous pPulmonary vVenous cConnection
- d-TGA
- Tricuspid Aatresia
- Truncus aArteriosus
CCHD screening is less sensitive in detecting cardiac lesions that may not present with early hypoxemia. These infants may pass the CCHD screen and be discharged after an unremarkable and brief birth hospitalization.
The secondary screening targets of the CCHD screen are as follows:
- Coarctation of the aorta
- Double outlet right ventricle
- Ebstein's Anomaly of the tricuspid valve
- Interrupted Aortic Arch
- Single ventricle
Special Considerations for Eisenmenger Syndrome:
In Eisenmenger syndrome is , i.e.a reversal of L R shunt due to pulmonary hypertension cyanosis. The following cascade may occur:
Chronic hypoxemia → polycythemia → immature and less malleable RBCs (micro-spherocytes) due to relative iron deficiency → microvascular occlusion → stroke
Patients with chronic cyanosis due to Eisenmenger syndrome may require special management:
- Adequate hydration
- Iron supplementation production of mature malleable RBC's
- Oxygen supplementation
- Aspirin
They may eventually require pulmonary vasodilators and phosphodiesterase inhibitors, endothelial receptor blockers or even heart-lung transplantation.
References:
- Dasgupta, S., Bhargava, V., Huff, M., Jiwani, A. K., & Aly, A. M. (2016). Evaluation of The Cyanotic Newborn: Part I—A Neonatologist's Perspective. NeoReviews, 17(10), e598-e604.

